These are complex ions in which the central metal ion is forming six bonds. Cis- represents that the same ligands are next to each other ie.
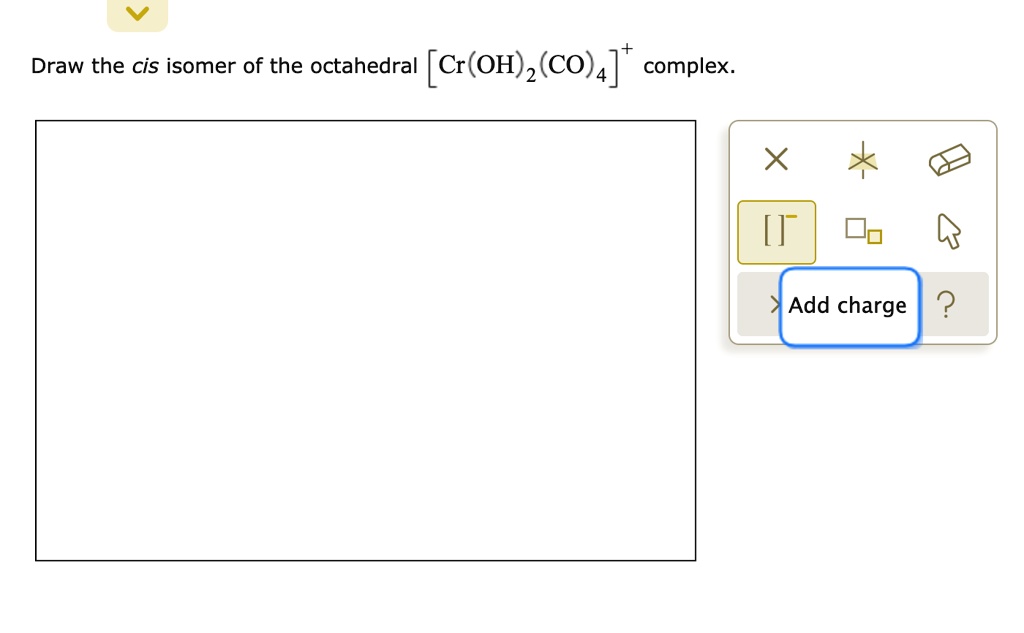
Solved Draw The Cis Isomer Of The Octahedral Cr Oh 2 C0 4 T Complex Add Charge
At 180 degrees relative to the central metal ion.

. Draw the cis isomer of the octahedral CrCl 2 CO 4 complex. Cis ligands and drawing the picture. Go to Animation SC171.
Draw the cis and trans isomers of CoNH 3 4 Cl 2. Draw the possible geometric isomers. Cis Trans Isomers of DichlorobisethylenediaminecobaltIII Chloride Introduction Geometrical isomers are metal complexes in which the coordinating ligands are present in the same proportion but vary in the arrangement of.
Define cis and trans isomerism. Like square planar complexes only one structure is possible for octahedral complexes in which only one ligand is different from the other five MA 5 B. Even though we usually draw an octahedron in a way that suggests that the four in-plane ligands are different.
How many isomers are possible for a tetrahedral complex ma2bc. Four of the ligands are in one plane with the fifth one above the plane and the sixth one below the plane. The cis-isomer of MAA 2 b 2 may also exhibit optical isomerism although we will concentrate largely on optical isomers of the type MAA 3 see below.
At 90 degrees in relation to the central metal ion whereas in the trans- isomer they are opposite each other ie. Octahedral complexes also exhibit cis and trans isomers. Get the answer to your homework problem.
Solution for Draw all the isomers of an octahedral complex having four different monodentate ligands. Then the other ligands can have cis or trans arrangements on the plane perpendicular toi the axis of the first A and B ligands. Facial and meridional isomers are possible with respect to the octahedral complex.
The CoIII has two geometric isomers available. Weve got the study and writing resources you need for your assignments. In coordination isomerism both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands between the cation and the anion.
Start your trial now. Facial 3 ligands occupy the face of an octahedron. All Questions Ask Doubt.
A three-dimensional model of cis-NH 3 4 CoCl 2. Meridional where 2 of the ligands are trans and the third is cis. Dien is the linear tridentate ligand NH 2 CH 2 CH 2 NHCH 2 CH 2 NH 2.
Linkage isomerism occurs with ambidentate ligands that can bind in more than one place. 25 Draw the cis and trans isomers of the octahedral complex CrBr-HO Be sure to label which is which. Does cis-amminebromo-cis-chloropyridineplatinumII uniquely identify isomer 9ii.
Octahedral complexes can also have two particular ligands adjacent to each other or on opposite sides of the metal atom. 𝕍𝔹𝕋 𝕠𝕗 ℂ𝕠ℂ2𝕆43-3. A tetrahedral arrangement symbolized as Mabcd four different ligands consists of two possible optical isomers.
Experts are tested by Chegg as specialists in their subject area. Click hereto get an answer to your question Draw cis and trans isomers of CoCl2NH34Cl. In the simple cases we are talking about that means that it will be attached to six ligands.
These ions have an octahedral shape. For example the cation NH 3 4 CoCl 2 has a cis-isomer and a trans-isomer. For example NO 2 can bind to a metal at either the N atom or an O atom.
Draw the geometric isomers of the octahedral complex CodienBr 2 Cl. If we first draw an A ligand and a B ligand opposite one another in an octahedral geometry. We review their content and use your feedback to keep the quality high.
The octahedral arrangement symbolized by Ma 2 b 2 c 2 where the three pairs of ligands are all cis with respect to each other has two optical isomers. Cis- and trans-isomers of an octahedral cobalt compound. MA-A 3 has optical isomers.
Who are the experts. Is it the same isomer. First week only 499.
The suffix a t e shows its an anionic ligand complex. Answered by 4th Jun 2014 0323. Of isomers Ma 4b2 2 cis- and trans- Ma 3b3 2 fac- and mer- MAA 2b2 3 2cis- and 1 trans- here a and b are monodentate ligands and AA is a bidentate ligand see Figures 10 11 12 in the Handout.
Octahedral complexes also exhibit cis and trans isomers. Optical Isomerism for Some Common Octahedral Complex Compositions 1. Octahedral complexes with the general formula MA_3B_3 exhibit mer-fac isomerism.
You can avoid drawing repeat structures if you switch just two ligands at a time rather than randomly drawing a new structure Use the method for describing the complexs ligands as given in 2. Optical Isomers Optical isomers are related as non-superimposable mirror images and differ in the direction with which they rotate plane-polarised light. Like square planar complexes only one structure is possible for octahedral complexes in which only one ligand is different from the other five MA 5 B.
Octahedral complex CoNH 3 4 Cl 2 exists as cis and trans isomer. A cis-dichlorotetracyano-chromate III. Even though we usually draw an octahedron in a way that suggests that the four in-plane ligands are different from the two.
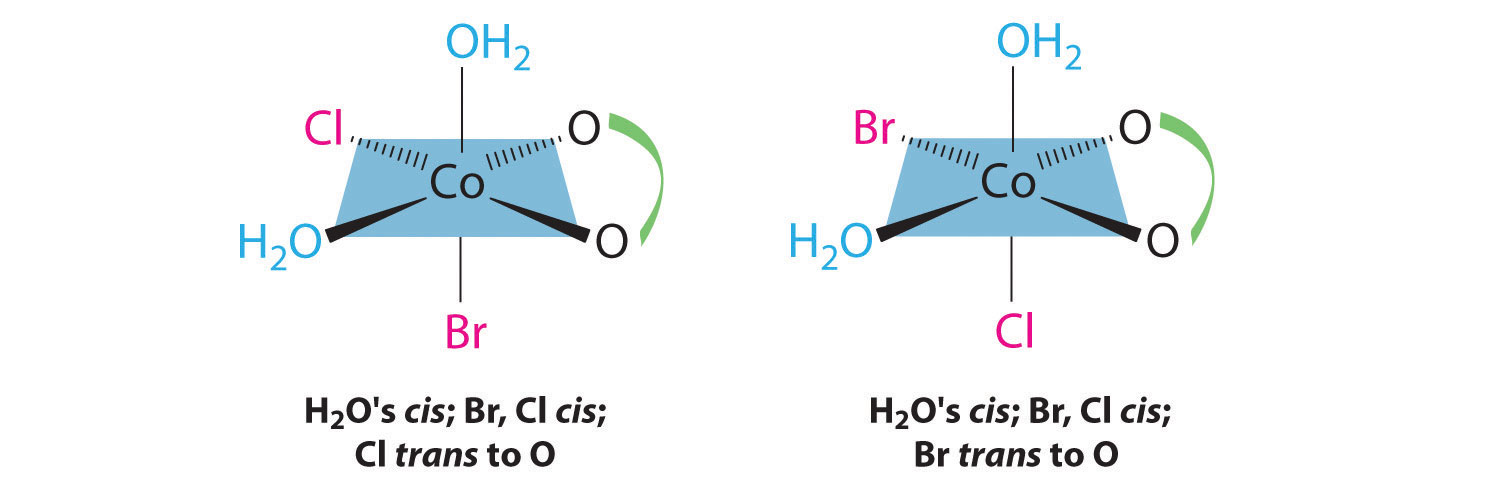
Stereoisomers Geometric Isomers In Transition Metal Complexes Chemistry Libretexts
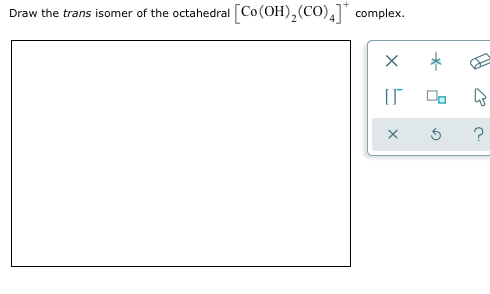
Solved Draw The Trans Isomer Of The Octahedral Chegg Com
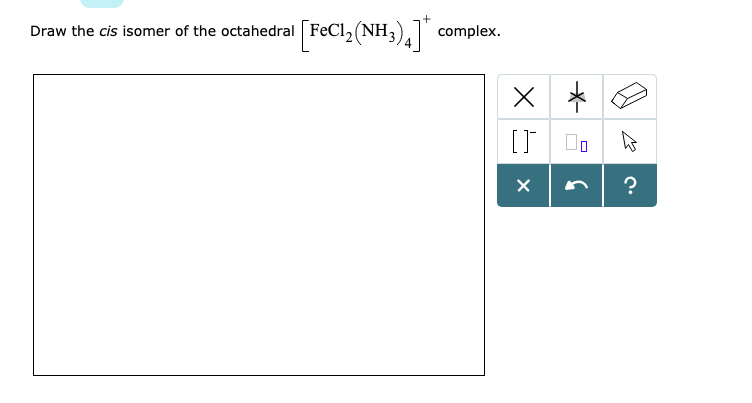
Solved Draw The Cis Isomer Of The Octahedral Fecl2 Nh Chegg Com
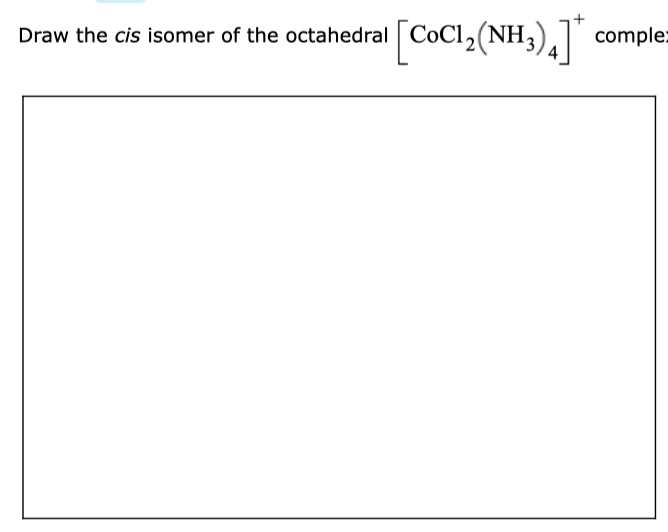
Solved Draw The Cis Isomer Of The Octahedral Cocl2 Nh3 4 Chegg Com

Solved Draw The Cis Isomer Of The Octahedral Crf Nh Chegg Com
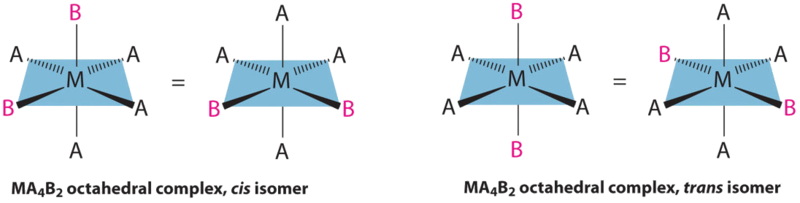
24 4 Isomerism In Coordination Complexes Chemistry Libretexts
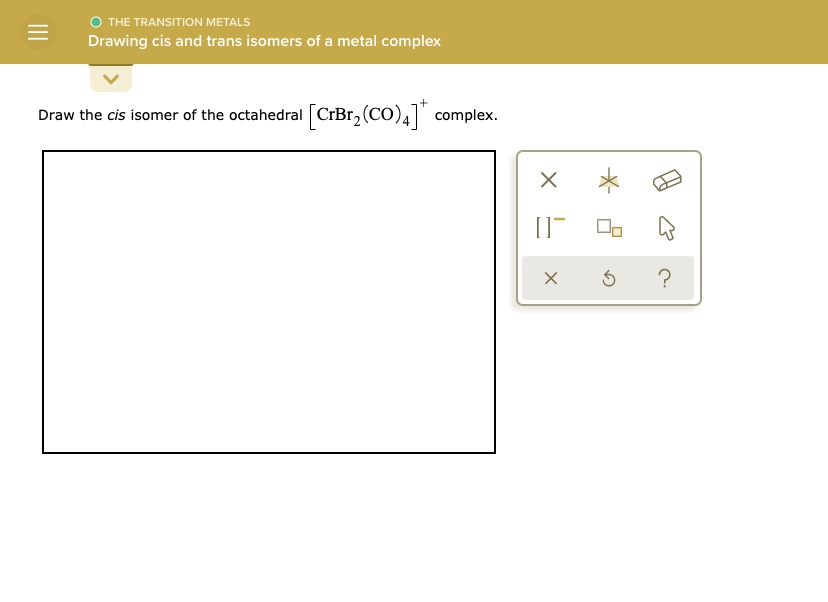
Solved The Transition Metals Drawing Cis And Trans Isomers Of A Metal Complex Draw The Cis Isomer Of The Octahedral Crbrz C0 4 Complex J
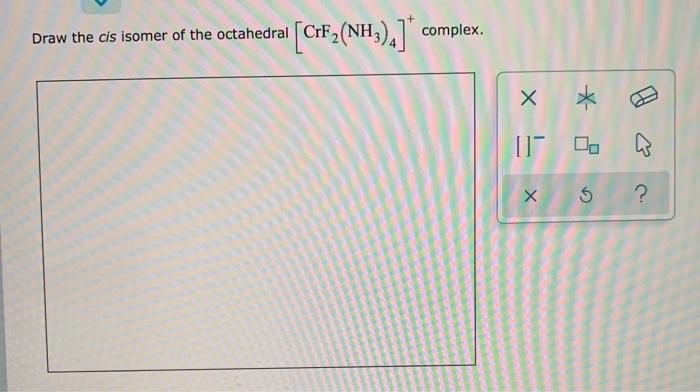
Solved Draw The Cis Isomer Of The Octahedral Crf Nh Chegg Com
0 comments
Post a Comment